Our review paper on the role of time in governing hydrogel mechanical properties is now out in Chemical Reviews. This comprehensive review was contributed to by current MMD lab members Zach Bernheimer and Si Chen as well as MMD alum Hongyi Cai. Many thanks to Prof Ben McDonald at Brown University for leading this effort! The review spans bond level and architecture level perspectives in the field, and especially highlights how far from equilibrium architectures can endow novel properties.
Hybrid Living Coatings to Detect Structural Damage
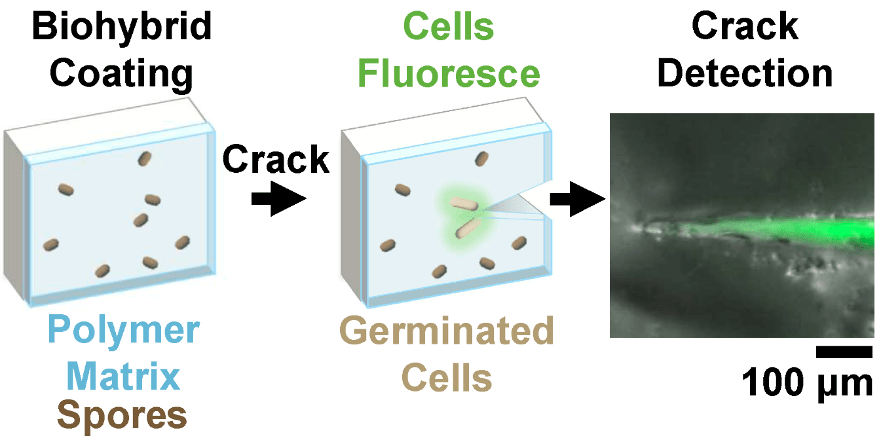
“Mechanically Driven Bacteria‐Based Crack Detection” led by PhD candidate Ellen van Wijngaarden is now published in RSC Materials Advances! In a collaborative work with the Giometto, Brito, and Bouklas labs at Cornell, we created a biohybrid coating that embeds bacterial spores into a polymer layer, enabling the material to sense and respond to damage for crack detection and mitigation. The system responds directly to mechanical damage across different materials and loading conditions, showing how living components can transform traditionally inert structures into responsive materials, to improve safety and reduce material waste. This work highlights the potential for engineered living materials for earlier damage detection for longer-lasting and responsive infrastructure.
Welcome to Zoë Masters
Welcome to Mechanical Engineering PhD candidate Zoë Masters – the newest addition to the MMD lab (officially joined in December)! Zoë will be focusing on experimental mechanics for both living and synthetic materials.

Living Architecture!
Ellen van Wijngaarden and Meredith Silberstein have a new paper out in Materials Advances on scalable manufacturing with freeform deposition of mycelium-bound composites. This work is in collaboration with the Wisniewska lab in Cornell’s Architecture department.

MMD Lab at SES
MMD lab members Ellen van Wijngaarden, Si Chen, Jaehee Lee, and Meredith Silberstein all presented at the Society of Engineering Science annual technical meeting in Atlanta last week. The team also caught up with MMD alumni Rob Wagner (now faculty at Binghamton University) and Allison Rzepka (now a PhD student at University of Illinois)!


New Paper in Nature Communications
Our lab is excited to share that Dr. Si Chen, ELMI and Fleming Postdoctoral Fellow, is the first author of our new paper, “Fibrous network nature of plant cell walls enables tunable mechanics for development,” published in Nature Communications.
This interdisciplinary study, at the interface of solid mechanics and plant developmental biology, was made possible through collaborations fostered by the Engineered Living Materials Institute (ELMI).
In this work, we show that plant primary cell walls behave like nonlinear fibrous networks, allowing them to stretch, reorient, and adapt their structure. Using both experiments and modeling, we uncovered the deformation mechanisms behind this behavior, examined how cell wall mechanics change during growth, and explored a mutant with altered leaf shape.
Together, these findings reveal how the fibrous architecture of cell walls provides tunable mechanical properties, enabling plants to adjust growth in ways that support proper development.
Congratulations to Si and the team for this exciting step toward engineering living systems into functional, sustainable materials! Special shout out to co-author and former MMD undergrad Bex Pendrak (now a Mechanical Engineering PhD candidate at Columbia University) for initiating this collaboration.
Advancing Ionic Circuits!
A new paper, “Harnessing ionic complexity: A modeling approach for hierarchical ionic circuit design” is now out in APS Physical Review Applied and was led by Dr. Max Tepermeister. In this work, we developed a flexible circuit-like model for designing and understanding the behavior of ionic circuitry. Our model allows designers to approach ionic circuitry from a materials focused or device focused perspective, and gives new insights into transient behavior, circuit memory, and circuit stability; all while solving ionic circuits many times faster than realtime. We have also released our work as an open-source library for the modelica systems language, which we hope the ionic community will build on.
Regulating Hydrogel Stiffness with Electric Fields
Work led by the newly minted Dr. Cai is now published in Materials Horizons. In “Regulating hydrogel mechanical properties with an electric field,” Dr. Cai designs a gel that is stable despite having strong saloplasticity (getting 5x stiffer when all of the salt is removed) and then shows how external electric fields can be used to regulate stiffness by regulating salt transport. He then demonstrates how this innovation could be applied for haptic devices. Max Tepermeister helped with system design and a special thanks co-author to Chenyun Yuan from Prof Ober’s group for sharing his scanning electron microscopy expertise.


